- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
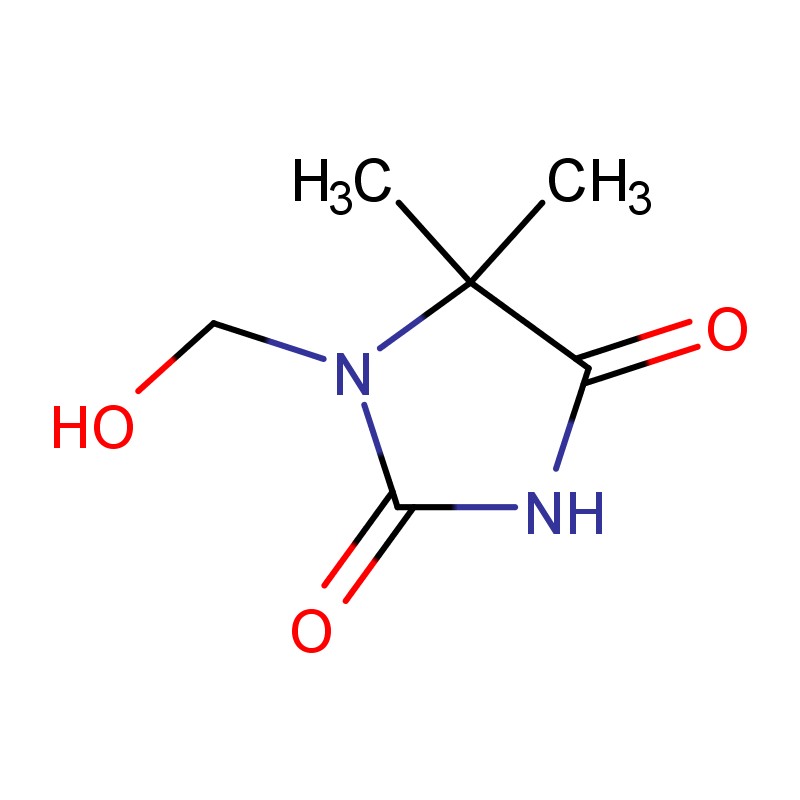
Why do Hydantoin Pharmaceutical Intermediates help me deliver faster and cleaner drug development?
I have managed enough tech transfers to know that speed without control is a trap. Working with the team at Leache, I keep choosing Hydantoin Pharmaceutical Intermediates because they balance reactivity with stability, give me tidy impurity maps, and scale without the usual surprises. Instead of wrestling with drift and rework, I get repeatable yields, sensible costs, and packaging that survives real supply chains. That is how I protect timelines and credibility.
Where do these intermediates fit best in real pipelines?
- I lean on Hydantoin Pharmaceutical Intermediates when I need robust heterocycles that tolerate a mix of electrophiles and nucleophiles across discovery and early scale
- I pick them for routes that favor clean crystallization windows and straightforward workups
- I use them when downstream targets require precise stereocontrol or tight impurity thresholds
- I deploy them to reduce solvent complexity and shorten cycle times in kilo labs and pilot plants
What makes hydantoin scaffolds so reliable in scale up?
- Balanced reactivity that supports selective transformations and reduces side reactions
- Thermal behavior that plays well with standard glass lined equipment and common utilities
- Predictable polymorph tendencies that simplify crystallization screening and filtration
- Consistent quality trajectories from grams to tons, which lowers the risk of revalidation
How do I evaluate a hydantoin supplier without missing hidden risks?
- Look for transparent impurity origin stories and not just a certificate of analysis
- Ask for reaction monitoring data that ties process controls to impurity outcomes
- Check scale history and whether batches have crossed critical volume gates without deviations
- Confirm packaging engineering and lane testing rather than relying on generic cartons
- Request stability snapshots under heat, humidity, and light to avoid late surprises
| Parameter | Typical value | Why it matters | Leache practice |
|---|---|---|---|
| Assay by HPLC | ≥ 99.0 percent | High assay supports shorter purification trains | Tight in process controls keep variability low |
| Total impurities | ≤ 0.5 percent | Cleaner feeds reduce cumulative burdens downstream | Route scouting targets impurity precursors at the source |
| Residual solvents | Meets ICH Q3C | Regulatory comfort and safer plant conditions | Validated purge steps and solvent qualification |
| Moisture | ≤ 0.5 percent | Water sensitive couplings run more reliably | Desiccant monitored storage with sealed liners |
| Batch size readiness | From kilos to multi ton | Fewer route changes during scale up | Parallel reactors and mirrored unit ops |
| Packaging | HDPE drums or FIBC | Mechanical and moisture protection in transit | Multi layer liners and lane qualification |
| Series focus | Hydantoin Pharmaceutical Intermediates | Core platform chemistry for reliability | Independent R and D with automated plants |
Which pain points do these intermediates solve for my team?
- They cut the number of solvent swaps and save hours on each cycle
- They shorten troubleshooting because impurity pathways are well understood
- They raise the chance that discovery routes remain viable in the plant
- They keep budget variance in check through steady yields and predictable consumables
- They let me present confident forecasts to stakeholders who care about launch windows
When I choose Hydantoin Pharmaceutical Intermediates, I lower operational noise and free energy for the steps that really need creativity.
How does Leache keep quality strong while costs stay sensible?
The value comes from pairing process knowledge with automation, in line analytics, and disciplined raw material control. The result is fewer deviations and a cleaner cost picture across the life of a program. I see it in the way batches land within spec without drama, and in the steadiness of lead times even when demand spikes.
When should I choose hydantoin over other heterocycles?
- When the route benefits from stable urea like backbones that accept diverse substitutions
- When the target demands narrow impurity windows and tight control of residuals
- When crystallization is the preferred workup and polymorph screening must be efficient
- When downstream steps are moisture sensitive and storage conditions need to be practical
In these settings, Hydantoin Pharmaceutical Intermediates give me a dependable platform that protects schedules.
What packaging and logistics choices protect every batch from plant to port?
- Drums with multi layer liners safeguard against humidity shifts and physical stress
- Lane testing for air and ocean confirms that packaging survives stacking and vibration
- Pallet patterns and corner protection reduce compression risk and keep labels scannable
- Documentation packs travel with the goods so receiving teams can release material fast
These details matter because Hydantoin Pharmaceutical Intermediates deserve the same care outside the reactor as inside it.
How can I start small and build momentum without heavy risk?
- Begin with sample quantities and route conversations that map impurities to controls
- Lock basic specs early and align on analytical methods to avoid friction later
- Use pilot batches to confirm equipment fit and cycle time targets
- Scale in planned steps so validation feels like a formality rather than a gamble
This path lets me qualify Hydantoin Pharmaceutical Intermediates with confidence while keeping capital and time commitments sensible.
What sets my procurement decision apart when the clock is ticking?
With Hydantoin Pharmaceutical Intermediates, I present cleaner data packages, steadier lead times, and fewer change controls. That is how I keep projects moving and reputations intact. It is also why I return to the same reliable platform when the next target lands on my desk.
Shall we plan your next campaign together?
If you are mapping a route or preparing a scale move and want a calmer path with fewer unknowns, tell me what you are building and what constraints you face. The team is ready to align on specs, samples, and timelines. If you are ready to request a quote or a technical call, please contact us today and let us know how Hydantoin Pharmaceutical Intermediates can support your program.