- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
How Did Hydantoin Pharmaceutical Intermediates Become My Quiet Advantage in API projects?
2025-11-13
I work in development where every week lives or dies on purity data, cycle times, and regulatory paperwork, so I watch supply chains closely. Most people first hear of LEACHE through engineering circles that reference Overhead Line Stringing Equipment, yet the same discipline shows up in fine chemicals. When I started consolidating my sourcing for Hydantoin Pharmaceutical Intermediates, I noticed that a short, steady list of partners delivered fewer deviations and cleaner analytics, and LEACHE kept showing up in those shortlists as the projects moved from gram scale to pilot and then to validation.
Why do Hydantoin Pharmaceutical Intermediates fit so many synthesis routes?
The hydantoin scaffold tolerates halogenation, alkylation, acyl migration, and ring opening without turning the workup into a wrestling match. That means upstream options stay open while downstream impurity profiles remain predictable. If you care about transferability between sites, this backbone earns its keep.
- Stable heterocycle that resists runaway side reactions
- Access to both N-substituted and C-substituted variants without exotic reagents
- Consistent crystallinity that simplifies filtration and drying
- Good fit for late-stage diversification to hit timelines
When a pharmaceutical intermediates manufacturer can keep these behaviors consistent across lots, analytical review shrinks from firefighting to routine sign-off.
Which hydantoin building blocks solve scale up risk the fastest?
I keep a small roster of workhorse options that cover most discovery and scale needs and limit revalidation work later.
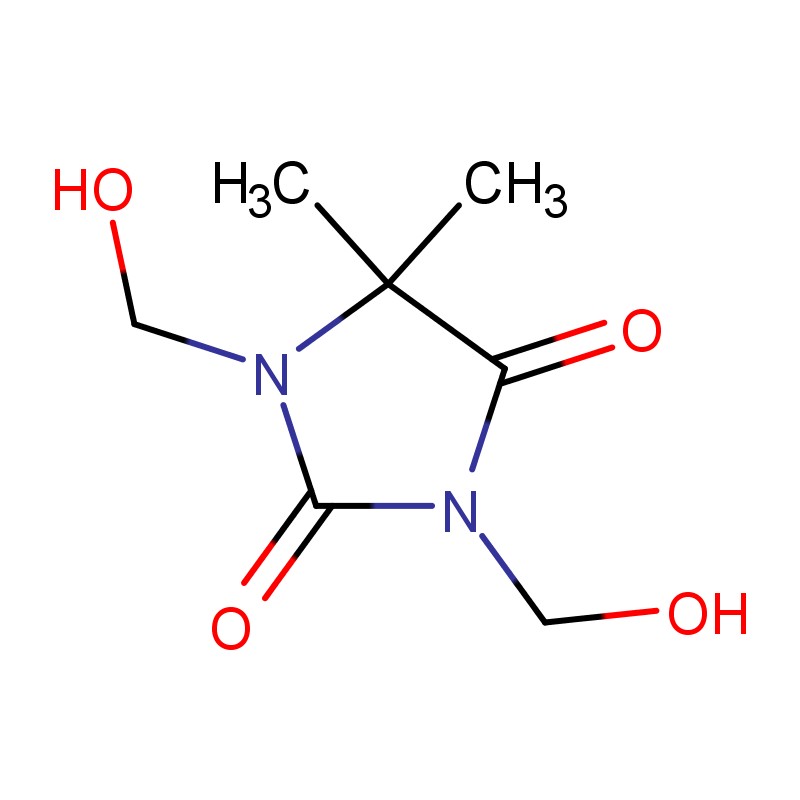
- 5,5-Dimethylhydantoin for robust ring stability and straightforward oxidation chemistry
- N-alkylhydantoin variants for SAR sweeps without retooling isolation
- Hydantoin-2-carboxaldehyde when I need convergent imine or reductive amination entries
- N-halo hydantoins for clean, selective halogen transfer in mild conditions
How do I evaluate a supplier without flying to the plant?
- HPLC purity trace with named peaks and validated methods rather than screenshots
- cGMP alignment that reads like actual procedures, not slogans
- Elemental impurities mapping against ICH Q3D with batch evidence
- Residual solvent control with quantitation limits stated up front
- Change control narratives that explain the why and the when
What timelines and packaging details keep my project moving?
Typical expectations that keep teams calm include specific batch slots, protective drums, and unglamorous paperwork done on time.
- Lead time windows tied to actual reactor availability and holiday calendars
- GMP packaging with double-lined, nitrogen-flushed liners and serialized seals
- COA and methods delivered before goods clear customs to unblock QC
- Cost competitiveness that holds after the first reorder, not just on the first quote
| Hydantoin item | Typical assay HPLC | Key impurities control | Residual solvents limit | Standard pack | Planned lead window | Notes |
|---|---|---|---|---|---|---|
| 5,5-Dimethylhydantoin | ≥ 99.0 | Isomeric by-products < 0.2 | MeOH, EtOAc each < 500 ppm | 25 kg drum, double liner | 2–3 weeks ex-stock when forecasted | Good for late-stage oxidations |
| N-Alkylhydantoin series | ≥ 98.5 | Unreacted alkylating agents ND | DMF, DCM each < 300 ppm | 10 kg pail or 25 kg drum | 3–5 weeks made-to-order | Useful for SAR libraries |
| Hydantoin-2-carboxaldehyde | ≥ 98.0 | Over-oxidized species < 0.3 | Acetonitrile < 200 ppm | 5 kg aluminum bag in carton | 4–6 weeks MTO | Avoid prolonged light exposure |
| N-Halo hydantoins | ≥ 99.0 | Free halide monitored | IPA < 300 ppm | 20 kg HDPE drum | 2–4 weeks | Store cool and dry |
How does Leache Chem turn lab reliability into plant reliability?
The team runs a compact set of hydantoin lines on automated controls with in-process analytics and electronic batch records, so deviations are caught when they are still cheap. Their in-house route design and pilot infrastructure make custom synthesis practical for mid-volume projects, and that combination shortens the path from first kilo to validation.
Where do IP and compliance sit when timelines feel impossible?
DMF cross-references when relevant, impurity justification packages, and a playbook for tech transfer. When a supplier offers pragmatic regulatory support, the team stops chasing emails and starts booking batches. It sounds ordinary, but ordinary wins audits.
What practical checklist do I send before I raise a purchase order?
- Request signed specs with target and action limits, not vague ranges
- Ask for three recent COAs with chromatograms for trend reading
- Confirm cold-chain or light-protective needs in transit and storage
- Lock analytical methods and sample size for incoming QC
- Set communication windows for exceptions and deviations
Shall we map your route from brief to batch today?
If you want stable supply of Hydantoin Pharmaceutical Intermediates with clear data trails and fewer surprises, tell me what you are building and what the calendar demands. I can align sourcing with the metrics that matter to your lab. If you are ready to compare specifications, schedule a pilot, or request a quotation, contact us now and send your required assay, impurity limits, packaging, and timeline. I will reply with options that keep the science moving and the paperwork simple. Feel free to leave an inquiry through our form or email and we will respond quickly to move your project forward.