- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
What Makes N-Bromosuccinimide a Preferred Reagent in Organic Synthesis?
2025-10-14
N-Bromosuccinimide is a highly versatile brominating agent widely used in organic chemistry, pharmaceutical synthesis, and polymer chemistry. Its unique ability to selectively brominate allylic and benzylic positions, as well as participate in radical reactions, makes it indispensable for precise molecular modifications. This article explores the functionality, advantages, and future trends of NBS while providing detailed technical parameters to guide industrial and laboratory applications.
NBS operates under controlled conditions to deliver predictable results, offering safety and efficiency advantages over elemental bromine. With applications ranging from fine chemicals to large-scale pharmaceutical intermediates, understanding its properties, usage, and best practices is critical for researchers and manufacturers seeking reliable bromination reagents.
What Are the Key Properties and Applications of N-Bromosuccinimide?
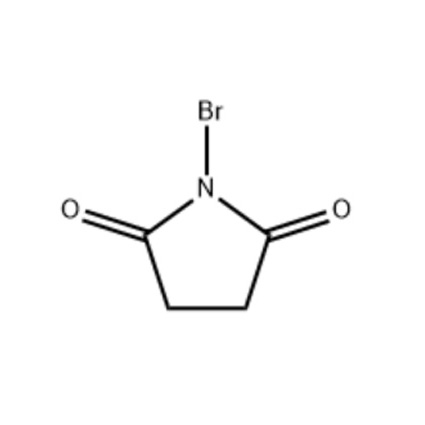
NBS is a white to off-white crystalline powder with high chemical stability under standard conditions. Its reactivity is primarily due to the N-Br bond, which facilitates selective bromination. The compound’s controlled release of bromine makes it safer and more manageable than direct halogenation with liquid bromine.
Key Technical Parameters of N-Bromosuccinimide:
| Parameter | Specification / Value |
|---|---|
| Molecular Formula | C4H4BrNO2 |
| Molecular Weight | 177.98 g/mol |
| Appearance | White to off-white crystalline powder |
| Purity | ≥ 98% |
| Melting Point | 173–175°C |
| Solubility | Soluble in water, acetone, chloroform, DMF |
| Stability | Stable at room temperature, sensitive to light |
| Applications | Allylic/benzylic bromination, radical reactions, synthesis of pharmaceuticals and fine chemicals |
Primary Applications:
-
Organic Synthesis: Selective bromination at allylic and benzylic positions.
-
Pharmaceutical Industry: Intermediate for drug synthesis and active pharmaceutical ingredients (APIs).
-
Polymer Chemistry: Functionalization of polymer chains for improved material properties.
-
Laboratory Reagent: Radical bromination reactions under controlled conditions.
The versatility of NBS has made it a standard choice for chemists seeking precision in functional group transformations. Compared to traditional bromine, NBS provides reduced hazards, easier handling, and cleaner reaction profiles.
Why Is N-Bromosuccinimide Preferred Over Other Brominating Agents?
The growing demand for safer, more efficient bromination reagents has positioned NBS as a preferred choice in modern chemistry. Unlike elemental bromine, which poses handling hazards and uncontrolled reactions, NBS allows controlled release of bromine under mild conditions. This provides high selectivity and minimal side reactions.
Advantages of NBS:
-
Selective Bromination: NBS selectively targets allylic, benzylic, and other activated positions without over-bromination.
-
Controlled Reactivity: Gradual bromine release reduces risks associated with highly reactive halogens.
-
Versatility: Effective in various solvents and reaction media including aqueous, organic, and polar aprotic solvents.
-
Stability and Storage: NBS is solid at room temperature and easier to store compared to volatile bromine solutions.
-
Environmental and Safety Profile: Reduced bromine vapor and exposure risk, making it compliant with modern safety standards.
Future Trends:
-
Green Chemistry Integration: Development of eco-friendly bromination methods using NBS in solvent-free or aqueous systems.
-
Pharmaceutical Expansion: Increased use in synthesizing novel APIs and complex drug intermediates.
-
Automated and Continuous Flow Synthesis: Incorporation into automated reaction setups to enhance reproducibility and scale-up potential.
NBS has demonstrated a consistent record of reliability, making it a reagent of choice for laboratories and industrial-scale manufacturers aiming for high efficiency and safety in bromination reactions.
How Should N-Bromosuccinimide Be Used for Optimal Results?
Proper usage of NBS is critical to maximize its efficiency and ensure safety. The reagent’s performance depends on reaction conditions, solvent choice, temperature control, and the presence of radical initiators.
Best Practices for NBS Application:
-
Solvent Selection:
-
Polar aprotic solvents like DMF or DMSO are recommended for enhanced solubility and reaction control.
-
Non-polar solvents like carbon tetrachloride may be used in radical bromination with light initiation.
-
-
Temperature Control:
-
Conduct reactions at moderate temperatures (0–40°C) to prevent decomposition or side reactions.
-
-
Initiators and Catalysts:
-
Radical initiators such as benzoyl peroxide or AIBN can enhance allylic or benzylic bromination.
-
-
Safety Measures:
-
Avoid direct contact with moisture and light.
-
Store in tightly closed containers under cool, dry conditions.
-
Use proper PPE when handling powders and solutions.
-
Common Questions About NBS Usage:
Q1: Can NBS be used in aqueous reactions?
A1: Yes, NBS is partially soluble in water, making it suitable for certain aqueous-phase bromination reactions. However, reaction efficiency may vary depending on substrate solubility and pH. Controlled addition is recommended to prevent uncontrolled release of bromine.
Q2: How can side reactions be minimized when using NBS?
A2: Minimize side reactions by carefully controlling reaction temperature, solvent choice, and stoichiometry. Avoid excess NBS to reduce polybromination, and use radical initiators judiciously for allylic or benzylic bromination.
Optimized usage of NBS ensures high product yield, precise bromination, and safe laboratory operations.
What Are the Future Developments and How Leache Provides High-Quality NBS?
The market for N-Bromosuccinimide continues to expand due to increased applications in pharmaceuticals, polymers, and fine chemical synthesis. Future developments include:
-
Green and Sustainable Bromination: Eco-friendly solvents and low-energy processes.
-
Enhanced Formulations: Modified NBS with improved solubility and reactivity profiles for specific industrial applications.
-
Automation in Chemical Manufacturing: Integration of NBS into continuous flow reactors to enhance scalability and consistency.
Leache stands as a trusted provider of high-quality N-Bromosuccinimide, ensuring purity above 98% and adherence to international standards. With comprehensive technical support and consistent product reliability, Leache enables chemists and manufacturers to achieve optimal results while maintaining safety and efficiency.
For inquiries about our N-Bromosuccinimide and detailed product specifications, contact us to receive guidance and order information tailored to your industrial or laboratory needs.