- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
Why Should Chemists Choose 2-Bromothiophene for Their Reactions?
2025-09-26
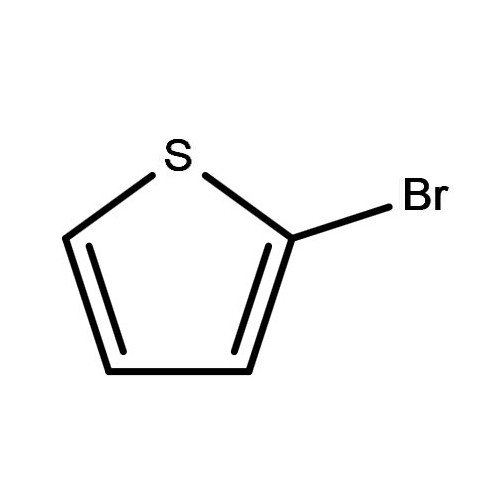
2-Bromothiophene is a critical heterocyclic compound widely used in the synthesis of pharmaceuticals, agrochemicals, and advanced materials. Its molecular structure, featuring a bromine atom substituted at the 2-position of the thiophene ring, makes it highly reactive and versatile for a variety of chemical transformations.
The core utility of 2-Bromothiophene lies in its ability to participate in cross-coupling reactions such as Suzuki, Stille, and Negishi couplings. These reactions are essential for constructing complex molecules in organic chemistry with high precision. Unlike other halogenated thiophenes, 2-Bromothiophene balances reactivity and stability, ensuring that it can be safely handled in laboratory and industrial environments without excessive decomposition.
From an industrial perspective, the compound serves as a building block in the development of conductive polymers and organic semiconductors. This makes it a key component for innovations in electronic materials, including OLED displays, photovoltaic cells, and flexible electronic devices. Understanding the chemistry of 2-Bromothiophene and leveraging its unique properties allows researchers to design more efficient synthetic pathways and reduce unwanted by-products.
Moreover, the compound's compatibility with a wide range of solvents, such as DMF, THF, and toluene, enables flexibility in synthetic strategies. Its solubility profile ensures that reactions proceed efficiently, providing high yields and reproducibility across different scales of production. This makes 2-Bromothiophene not only a laboratory favorite but also a reliable choice for large-scale industrial synthesis.
How Does 2-Bromothiophene Function in Organic Synthesis?
One of the key questions chemists ask is: how does 2-Bromothiophene enhance reaction efficiency and selectivity? The answer lies in its electronic properties and steric arrangement. The bromine atom in the 2-position increases the electrophilicity of the ring, making it more susceptible to nucleophilic attack and facilitating the formation of C-C or C-N bonds in cross-coupling reactions.
Reaction Applications:
-
Suzuki Coupling: Enables the formation of biaryl compounds with high yield and selectivity.
-
Stille Coupling: Provides a route for attaching organostannanes to the thiophene ring.
-
Negishi Coupling: Facilitates the reaction with organozinc reagents for advanced molecule construction.
In addition, 2-Bromothiophene’s controlled reactivity allows chemists to fine-tune reaction conditions, optimizing temperature, catalyst selection, and solvent choice to minimize side reactions. This is particularly crucial in the synthesis of pharmaceuticals where purity and structural integrity are paramount.
The compound is also favored in heterocyclic chemistry for the construction of fused ring systems. By strategically using 2-Bromothiophene, chemists can introduce thiophene units into larger frameworks, which is essential for the development of biologically active molecules and functional materials. The controlled reactivity reduces over-substitution and allows precise modification of the target molecules.
Product Parameters:
| Parameter | Specification |
|---|---|
| Chemical Name | 2-Bromothiophene |
| Molecular Formula | C4H3BrS |
| Molecular Weight | 157.03 g/mol |
| Appearance | Colorless to light yellow liquid |
| Purity | ≥99% |
| Boiling Point | 154–156°C |
| Density | 1.53 g/cm³ |
| Solubility | Soluble in organic solvents (THF, DMF, Toluene) |
These parameters make 2-Bromothiophene suitable for both lab-scale synthesis and large-scale industrial applications, ensuring consistency, reproducibility, and safety in handling.
Why Is 2-Bromothiophene Preferred Over Other Halogenated Thiophenes?
Choosing the right thiophene derivative can significantly impact the efficiency of synthetic processes. So, why do chemists prefer 2-Bromothiophene over alternatives like 3-bromothiophene or 2-iodothiophene?
1. Reactivity Balance:
While 2-iodothiophene is more reactive, it is also more expensive and less stable. Bromine provides an ideal balance, offering sufficient reactivity for cross-coupling while maintaining manageable handling and storage conditions.
2. Structural Selectivity:
Substitution at the 2-position directs reactions in a predictable manner, allowing selective functionalization. This precision is critical for synthesizing complex molecules with high yields and minimal side products.
3. Cost-Effectiveness:
2-Bromothiophene is relatively affordable compared to iodinated analogs. For industrial-scale applications, this cost difference can significantly impact production budgets without compromising quality.
4. Versatility:
The compound’s compatibility with various catalysts, solvents, and reaction conditions makes it adaptable to multiple synthetic strategies. Whether in medicinal chemistry, materials science, or agrochemical synthesis, 2-Bromothiophene remains a preferred choice.
Common FAQs About 2-Bromothiophene:
-
Q1: Is 2-Bromothiophene safe to handle?
A1: Yes, when handled according to standard laboratory safety protocols. It should be stored in a cool, dry place, and personal protective equipment is recommended during handling. -
Q2: What solvents are ideal for reactions with 2-Bromothiophene?
A2: Common solvents include THF, DMF, and toluene, which offer excellent solubility and support high reaction efficiency. -
Q3: Can 2-Bromothiophene be used for large-scale industrial synthesis?
A3: Absolutely. Its high purity, stability, and cost-effectiveness make it suitable for both small-scale laboratory and large-scale industrial applications.
These FAQs address the most common concerns among chemists, providing clear and practical guidance for safe and effective use.
How to Optimize the Use of 2-Bromothiophene in Your Projects
Optimizing 2-Bromothiophene usage requires understanding its chemical properties, storage requirements, and reaction behavior. Proper storage at low temperatures and protection from moisture ensures the compound remains stable over time. In synthetic applications, selecting the right catalyst and solvent combination is key to maximizing reaction efficiency.
For pharmaceutical synthesis, controlling stoichiometry and reaction time reduces unwanted by-products, leading to purer final products. In materials science, leveraging its reactivity in cross-coupling reactions allows the creation of functionalized polymers with enhanced electronic properties. Researchers can adjust reaction conditions to achieve specific molecular architectures, demonstrating the compound's versatility.
Finally, the reliability of suppliers is a critical factor in consistent performance. Leache provides high-quality 2-Bromothiophene with consistent purity and comprehensive technical support. Their products are tailored to meet the demands of modern laboratories and industrial facilities. To discuss your requirements or place an order, contact us today for professional guidance and supply solutions.