- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
Why Is 2-Thiophene Aldehyde Essential in Chemical Synthesis?
2025-09-09
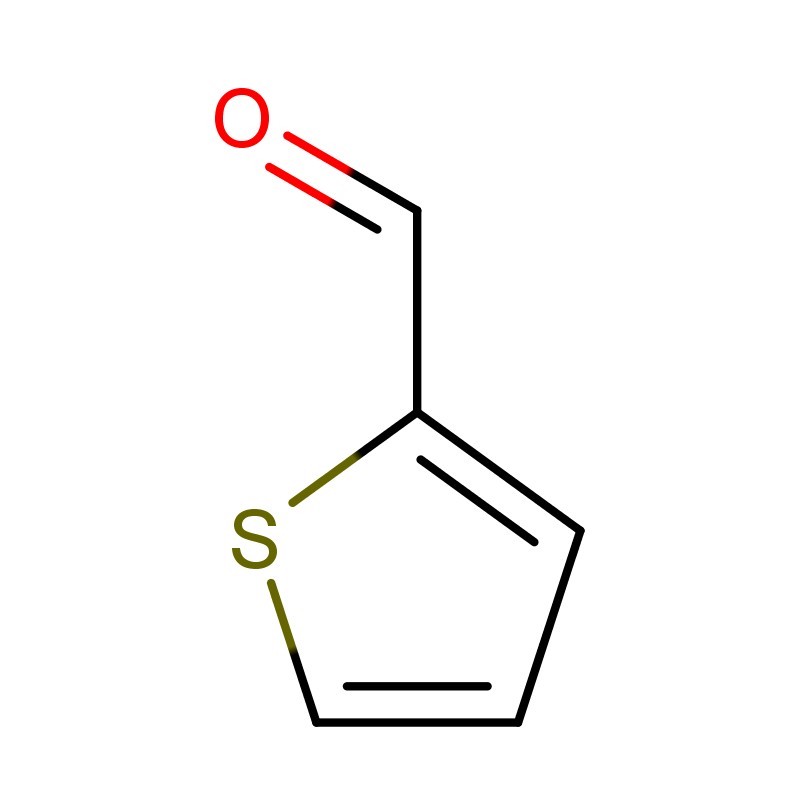
2-Thiophene Aldehyde, also known as thiophene-2-carboxaldehyde, is an aromatic heterocyclic compound widely used in pharmaceuticals, agrochemicals, and advanced material synthesis. With the molecular formula C₅H₄OS and a molecular weight of 112.15 g/mol, it plays a critical role as a versatile intermediate in multiple industries. Its unique structure, featuring a thiophene ring substituted by an aldehyde group, allows for highly selective reactions, making it valuable in organic synthesis and fine chemical manufacturing.
Key Physical and Chemical Properties
| Property | Specification |
|---|---|
| Chemical Name | 2-Thiophene Aldehyde |
| CAS Number | 98-03-3 |
| Molecular Formula | C₅H₄OS |
| Molecular Weight | 112.15 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Boiling Point | 217 °C (approx.) |
| Melting Point | -16 °C |
| Density | 1.24 g/cm³ |
| Purity | ≥99% (industrial & laboratory grade) |
| Solubility | Soluble in organic solvents, slightly soluble in water |
2-Thiophene Aldehyde’s high purity and controlled reactivity make it an indispensable intermediate in the synthesis of drugs, dyes, and agricultural formulations. Its electron-rich thiophene ring is particularly favorable for substitution and condensation reactions, which are commonly used in pharmaceutical and polymer manufacturing.
Applications of 2-Thiophene Aldehyde Across Industries
The demand for 2-Thiophene Aldehyde has grown steadily due to its broad industrial applications. From drug development to material science, its versatility makes it an essential compound for advanced chemical synthesis. Below are the major sectors where it plays a significant role:
a) Pharmaceutical Industry
2-Thiophene Aldehyde is a critical intermediate for manufacturing active pharmaceutical ingredients (APIs). It is frequently used in the synthesis of antiviral, antifungal, and anti-inflammatory compounds. Its structure allows for functionalization, making it a preferred starting material in heterocyclic drug design.
For example:
-
Cardiovascular drugs — used in compounds that regulate blood pressure and cholesterol.
-
CNS agents — acts as a precursor in antidepressants and anticonvulsants.
-
Anticancer research — contributes to developing thiophene-based antitumor compounds.
b) Agrochemical Production
In agriculture, 2-Thiophene Aldehyde is used for synthesizing herbicides, fungicides, and insecticides. Its aldehyde functionality allows it to be converted into derivatives that act as selective growth regulators and pesticide intermediates, improving crop yield and pest resistance.
c) Material Science and Specialty Chemicals
Thiophene derivatives are widely utilized in organic electronic materials, including OLEDs, organic photovoltaics, and conductive polymers. 2-Thiophene Aldehyde, due to its reactive aldehyde group, serves as a building block for functionalized thiophenes that enhance electronic conductivity and thermal stability.
d) Fragrances and Fine Chemicals
Although not widely used in consumer fragrances, some derivatives of 2-Thiophene Aldehyde are incorporated into aromatic compounds and flavoring agents. Its mild sulfur-containing aroma contributes to specific niche formulations in the flavor and fragrance industry.
Why Choose High-Purity 2-Thiophene Aldehyde for Industrial Needs
When selecting 2-Thiophene Aldehyde for research, manufacturing, or product development, purity, consistency, and stability are critical. Impurities can significantly alter reaction outcomes, reduce yields, and impact product safety. Choosing high-quality material ensures better efficiency, cost-effectiveness, and regulatory compliance.
Key Advantages of High-Purity 2-Thiophene Aldehyde
-
Superior reaction control — high-purity compounds provide predictable chemical behavior, essential for scale-up production.
-
Regulatory compliance — pharmaceutical and agrochemical industries require strict adherence to purity standards.
-
Enhanced product stability — low impurity levels reduce unwanted by-products and degradation.
-
Cost efficiency — consistent quality reduces the need for additional purification and quality checks.
Storage and Handling Recommendations
To maintain optimal stability, 2-Thiophene Aldehyde should be:
-
Stored in tightly sealed containers away from light and heat.
-
Kept at room temperature or under controlled cool storage.
-
Handled with protective gloves and eyewear to ensure safety during usage.
FAQs About 2-Thiophene Aldehyde
Q1: What is the primary use of 2-Thiophene Aldehyde in pharmaceuticals?
A: 2-Thiophene Aldehyde serves as a vital intermediate in synthesizing heterocyclic compounds used for manufacturing drugs like anticonvulsants, antifungals, and anticancer agents. Its aldehyde group allows for efficient coupling and condensation reactions, enabling the creation of highly functional molecules essential for modern medicines.
Q2: How does the purity of 2-Thiophene Aldehyde affect industrial applications?
A: The purity level directly influences the reliability of chemical reactions and final product quality. In pharmaceutical and electronic material production, impurities can lead to unstable compounds, reduced yields, and safety concerns. Using ≥99% purity ensures consistency, minimizes defects, and complies with international standards.
2-Thiophene Aldehyde is a fundamental building block in pharmaceutical synthesis, agrochemical development, material science, and fine chemicals manufacturing. Its unique reactivity, high selectivity, and versatility make it indispensable for researchers and industries seeking advanced solutions.
At Leache, we provide high-purity 2-Thiophene Aldehyde tailored for diverse industrial needs, ensuring exceptional quality and consistent performance. Whether you are scaling up production or developing innovative applications, our materials are optimized for reliability and efficiency.
For bulk orders, technical specifications, or customized solutions, contact us today to explore how Leache can support your business with top-grade 2-Thiophene Aldehyde.